CDC advisers voted on an RSV antibody using incomplete and intentionally manipulated data hiding seizure and death risks
08/25/2025 / By Lance D Johnson
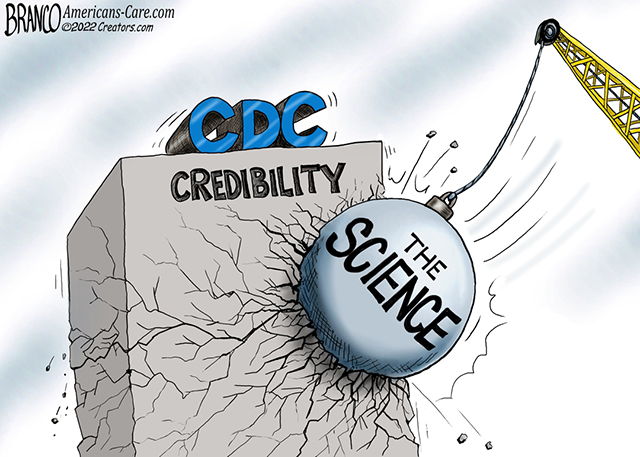
When the Advisory Committee on Immunization Practices (ACIP) goes to vote, the fate of millions of infants hangs on their advice. The advisers around the table are trusted experts, the data before them is supposed to be thorough, and the decision—whether to recommend a new medical treatment for every healthy newborn—carries irreversible consequences. Now imagine learning, months later, that the very data shaping that vote may have been distorted, that key safety signals were buried, and that the experts themselves were kept in the dark about critical risks. This isn’t a hypothetical scenario. It’s exactly what unfolded at an ACIP meeting in June 2025, when members voted to recommend Merck’s new RSV monoclonal antibody, clesrovimab, for widespread use in newborns.
The vote passed 5 to 2, but the foundation of that decision is now crumbling under the weight of new revelations. Independent analyses by investigative journalists and researchers suggest that the safety data presented to ACIP was incomplete, selectively framed, and in some cases, outright misleading. At the heart of the controversy is a troubling question: How could a committee tasked with protecting public health make such a consequential decision without seeing the full picture?
Key points:
- ACIP voted 5-2 to recommend Merck’s RSV antibody, clesrovimab, for all healthy newborns, despite dissent from members who cited unresolved safety concerns.
- Critical data on seizures and deaths was withheld or downplayed in the CDC’s presentation, including a fourfold increase in seizure risk when infant age groups were analyzed together—information never shown to the committee.
- Multiple clinical trials for RSV antibodies showed more deaths in treatment groups than controls, yet these imbalances were dismissed as “unrelated” without transparent breakdowns of causes or timing.
- Independent researchers found temporal clusters of infant deaths in France that aligned with nirsevimab distribution, a signal omitted from ACIP’s briefing.
- The CDC relied solely on one surveillance system (VSD) while ignoring VAERS and international data, creating a reporting blind spot that may hide adverse events.
- ACIP members now question whether they can trust future CDC presentations, with one adviser declaring he will no longer assume the data is “transparent, accurate, and unbiased.”
- The financial and legal protections for vaccine manufacturers—including immunity from liability—may incentivize rushed approvals and suppression of safety concerns.
The vote that wasn’t what it seemed
When Dr. Robert Malone, a member of ACIP, cast his vote in favor of recommending clesrovimab, he did so under the assumption that the data before him had been rigorously vetted. Like his colleagues, he had mere hours to review complex presentations the day before the meeting. What he didn’t know was that key safety signals had been fragmented, diluted, or omitted entirely.
The most glaring example? Seizure risk in infants.
During the meeting, ACIP members were shown data from the CDC’s Vaccine Safety Datalink (VSD), which tracked seizures in infants who received nirsevimab, a nearly identical RSV antibody already on the market. The data was split into two age groups:
- 0 to 37 days old: 3.5 times higher seizure risk (labeled “not significant”).
- 38 days to under 8 months old: 4.38 times higher seizure risk (also “not significant”).
But here’s the catch: When these two groups were combined—a standard practice in pharmacovigilance—the risk jumped to nearly four times higher, and it was statistically significant. This pooled analysis was never presented to the committee. Instead, the data was artificially divided at 38 days, the exact age when U.S. infants begin receiving routine vaccinations. By splitting the groups, the signal disappeared into statistical noise.
Dr. Maryanne Demasi, the journalist and researcher who uncovered this discrepancy, called it “a classic case of data slicing to obscure a safety signal.” The question is: Was this an honest oversight, or a deliberate attempt to mislead?
Dr. Malone, in a later blog post, didn’t mince words: “It appears that this decision was based on manipulated data analyses.”
The deaths no one wanted to see
If the seizure data was troubling, the mortality imbalances in clinical trials were downright alarming. Across four separate trials for nirsevimab and clesrovimab, one pattern emerged again and again: More infants died in the treatment groups than in the placebo groups.
- Nirsevimab trials: 12 deaths among 3,710 infants who received the antibody, compared to 4 deaths among 1,797 controls.
- Clesrovimab trials: 15 deaths in treatment groups versus 7 in controls.
In every single trial, the deaths skewed toward the infants who received the RSV antibody. Yet, in each case, investigators dismissed them as “unrelated”—often without providing detailed breakdowns of causes, timelines, or potential biological mechanisms.
Worse still, some deaths were buried in footnotes or excluded from analyses entirely. In one nirsevimab trial, a fifth death—occurring on Day 440—was omitted from the published safety data, even though the trial’s own protocol specified a 510-day follow-up window. Similarly, in Merck’s CLEVER trial, a death on Day 487 was mentioned in a footnote but not counted in the primary analysis.
Dr. Yaffa Shir-Raz, a risk communication researcher who analyzed the data, put it bluntly: “This isn’t just about transparency—it’s about integrity. When you hide deaths in footnotes, you’re not just obscuring data; you’re erasing lives.”
And then there’s the French data—a real-world red flag that never made it to ACIP.
In late 2023, researcher Hélène Banoun tracked infant deaths in France during the rollout of nirsevimab. She found statistically significant spikes in deaths among 2- to 6-day-old infants in September (55 deaths) and October (62 deaths)—months when the antibody was widely distributed. When supply constraints limited distribution in November, deaths dropped to 26. When distribution resumed, deaths climbed back up to 50 in December and 52 in January.
The pattern was too stark to ignore, yet the CDC’s presentation to ACIP made no mention of it.
A broken system now being held accountable by independent journalists
At its core, this controversy isn’t just about one vaccine or one meeting. It’s about a broken system where:
- Pharmaceutical companies enjoy near-total liability protection under the National Vaccine Injury Compensation Program, removing financial incentives to prioritize safety.
- Regulatory agencies like the CDC and FDA rely on the same industry-funded data they’re supposed to scrutinize, creating inherent conflicts of interest.
- Advisory committees like ACIP are given incomplete, cherry-picked information, leaving members to make billion-dollar decisions in the dark.
- Safety surveillance systems are fragmented, with RSV antibodies split between VAERS and MedWatch depending on how they’re administered—meaning adverse events can slip through the cracks.
- Dissent is punished. Doctors who question vaccine safety face licensing threats, while researchers who uncover inconvenient data are ignored or discredited.
Dr. Retsef Levi, one of the two ACIP members who voted against the RSV antibody recommendation, warned that “four different trials all show deaths going in the same direction.” Yet, instead of pausing to investigate, the committee pressed forward.
The fallout from this revelation is still unfolding. Dr. Malone has publicly stated he can no longer trust CDC presentations, and calls for reopening the vote are growing louder. But the damage may already be done: Clesrovimab is now part of the recommended schedule for infants, and nirsevimab is already in widespread use.
Sources include:
Submit a correction >>
Tagged Under:
ACIP vote, AstraZeneca, CDC corruption, clesrovimab, clinical trial deaths, data manipulation, FDA oversight, health freedom, infant deaths, medical ethics, medical transparency, Merck controversy, nirsevimab, Parental rights, pharmaceutical liability, public health fraud, RSV antibody, seizure risk, vaccine mandates, vaccine safety, VAERS failures
This article may contain statements that reflect the opinion of the author