Vaccine debate erupts: FDA chief questions hepatitis B shot for newborns
09/11/2025 / By Willow Tohi
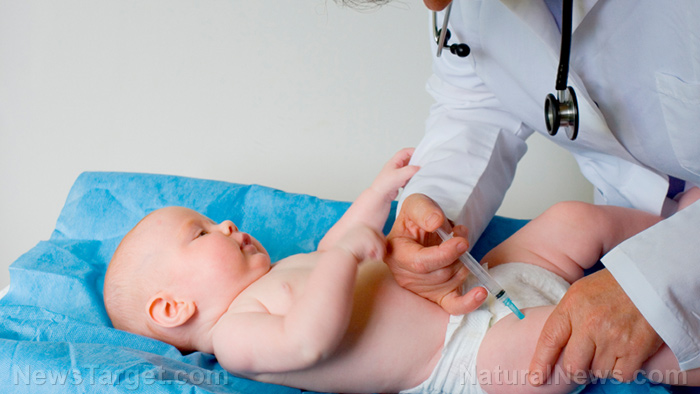
- FDA Commissioner Dr. Marty Makary questions the evidence supporting the CDC’s longstanding policy of administering the hepatitis B vaccine to newborns, calling it “not solid.”
- The CDC’s vaccine advisory panel will debate whether to alter or eliminate the universal birth dose requirement, with critics arguing most infants face minimal risk.
- Studies show hepatitis B vaccine protection declines significantly over decades, raising questions about the effectiveness of infant vaccination.
- Former CDC officials and lawmakers, including Sen. Roger Marshall (R-Kan.), argue for a risk-based approach, while others warn against relaxing recommendations.
- HHS Secretary Robert F. Kennedy Jr. is reshaping CDC leadership and vaccine policies, sparking resignations and concerns over ideological influence in public health decisions.
In a rare public dissent from longstanding federal health policy, FDA Commissioner Dr. Marty Makary declared this week that there is “no solid evidence” to support administering the hepatitis B vaccine to newborns—a practice mandated by the CDC since 1992. His remarks, made during a Fox News appearance, have ignited a fierce debate over vaccine schedules, scientific integrity and the influence of politics in public health.
Makary’s criticism comes as the CDC’s vaccine advisory committee prepares to review the policy, with former CDC vaccine chief Dr. Demetre Daskalakis predicting the panel may eliminate the universal birth dose requirement. The move would mark a dramatic shift in a policy that has been in place for over three decades, one that requires nearly all U.S. infants to receive the vaccine within 24 hours of birth.
“I personally don’t believe that the evidence is solid to say the Hep B shot needs to be given at birth,” Makary stated, contrasting it with “tried and true” vaccines like those for polio and measles. His comments reflect growing skepticism among medical professionals about the necessity of vaccinating all newborns, particularly when maternal screening already identifies high-risk cases.
The science behind the debate: Does infant vaccination work?
The hepatitis B vaccine was first recommended for high-risk groups in 1982 before expanding to all infants a decade later. While the vaccine has reduced childhood infections, critics argue that its long-term efficacy is questionable. A 2022 study in Vaccines found that among Thai medical students and healthcare workers vaccinated in infancy, only 28% retained protective antibodies after 16–20 years, with immunity declining at a rate of 42.39 mIU/mL per year.
“We’re giving about 11 million doses of hepatitis B a year, and pretty much all of them are for kids who we know don’t need it,” said Dr. Monique Yohanan of the Independent Women’s Forum, who advocates delaying vaccination until adolescence—when behavioral risks (such as sexual activity and IV drug use) increase.
The CDC acknowledges that antibody levels decline over time but maintains that “immune memory” may offer lasting protection. Yet, with no U.S. studies confirming lifelong immunity, some experts, including Dr. Martin Kulldorff, former chair of the CDC’s vaccine advisory committee, argue for a risk-based approach—reserving the birth dose for infants of hepatitis B-positive mothers.
Political fallout: Resignations, mandates and the Kennedy effect
The debate over hepatitis B is unfolding amid broader upheaval at the CDC, where HHS Secretary Robert F. Kennedy Jr. has fired or forced out top officials who resisted his policy changes. Earlier this month, CDC Director Susan Monarez was ousted after refusing to dismiss senior staff, while Dr. Daskalakis resigned, warning that the agency was “generating policies that do not reflect scientific reality.”
Kennedy’s influence extends to the Advisory Committee on Immunization Practices (ACIP), which he has restructured with appointees who share his skepticism of pharma-funded science, particularly mRNA technology. Daskalakis predicts the panel will target the hepatitis B birth dose next, potentially leaving infants of untested or high-risk mothers alone.
Sen. Roger Marshall (R-Kan.), an obstetrician, supports a more selective approach: “If [a mother] doesn’t have any risk factors… I don’t see the benefit myself in that hepatitis vaccine.” But Sen. Bill Cassidy (R-La.) counters that screening isn’t foolproof, and some at-risk mothers slip through the cracks.
The broader vaccine policy shift: What’s next?
Kennedy’s tenure has already led to major changes in COVID-19 vaccine recommendations, with the FDA restricting this fall’s updated shot to high-risk individuals and seniors—a decision that has sown confusion over insurance coverage and accessibility. Critics, including former acting CDC Director Dr. Richard Besser, warn that politicizing vaccine policy could erode public trust and leave the U.S. vulnerable to future outbreaks.
The hepatitis B debate is just one front in a larger war over vaccine mandates, scientific integrity and parental rights. Kennedy has signaled plans to announce new findings on autism and vaccines. Meanwhile, states like California have expanded vaccine mandates, while others are rolling them back, creating a patchwork of conflicting policies.
A crossroads for public health: Trust, transparency and the future
As the CDC’s advisory committee prepares to vote on the hepatitis B policy, the stakes extend beyond one vaccine. At issue is whether public health agencies will prioritize individualized risk assessment over universal mandates—and whether unbiased science can be used and trusted to determine safety and efficacy.
For parents, the shifting guidelines raise difficult questions:
- Should they trust decades-old policies, or demand more personalized medical advice?
- Will delaying vaccines increase long-term risks, or reduce unnecessary exposure to adjuvants like aluminum?
- Can the CDC regain credibility amid leadership turmoil and ideological divisions?
Dr. Besser’s advice remains clear: “Stop listening to the politicians, talk to your doctor about what’s right for you.” Yet, with trust in institutions at an all-time low, many Americans are left wondering whom to believe—and what the future of vaccine policy will look like under an administration that has openly questioned the science (funding) behind it.
The road ahead: A public health system in flux
The hepatitis B debate is a microcosm of a larger reckoning in American medicine. As the CDC grapples with leadership upheaval, ideological shifts and waning public trust, the decisions made in the coming months could reshape vaccine policy for generations.
One thing is certain: The era of unquestioned vaccine mandates may be ending. Whether that leads to greater medical freedom or preventable outbreaks remains to be seen. For now, parents, doctors and policymakers are navigating uncharted territory—where science, politics and personal belief collide.
Sources for this article include:
Submit a correction >>
Tagged Under:
. vaccines, awakening, bias, Big Pharma, CDC, Censored Science, FDA, health freedom, medical violence, pharma fraud, progress, Public Health, vaccine policy, vaccine wars
This article may contain statements that reflect the opinion of the author