Eli Lilly monoclonal antibody drug trial paused over safety concerns
10/20/2020 / By Franz Walker
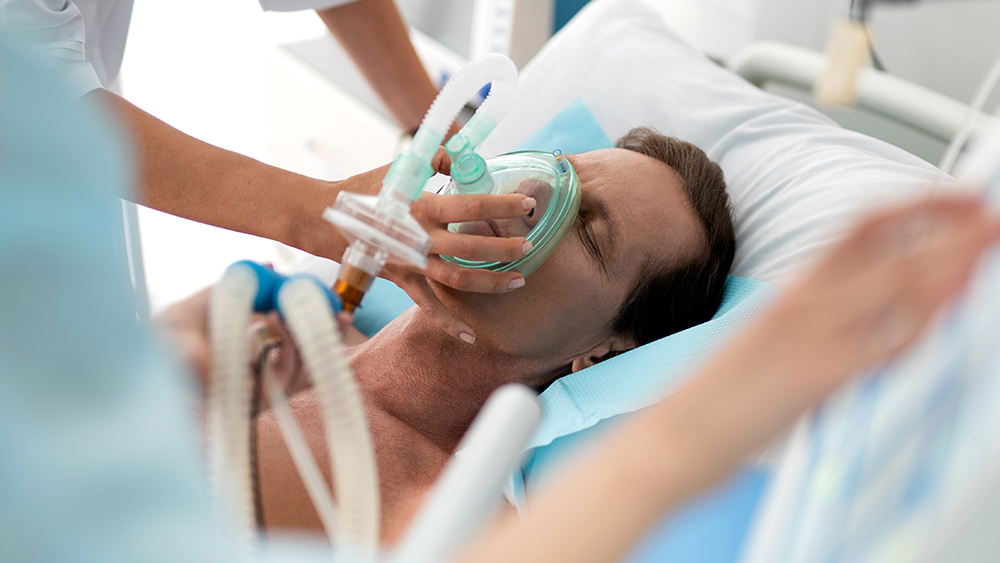
Eli Lilly & Company are halting the enrollment of participants in a clinical trial of its antibody treatment for the Wuhan coronavirus (COVID-19) due to safety concerns.
According to company spokeswoman Molly McCully, an independent data and safety monitoring board (DSMB) had recommended pausing enrollment in the U.S. government-sponsored trials. Lilly, however, did not say what caused the panel to recommend the pause.
“Safety is of the utmost importance to Lilly,” said McCully. “Lilly is supportive of the decision by the independent DSMB to cautiously ensure the safety of the patients participating in this study,” she added, referring to the independent panel of experts, known as the data and safety monitoring board.”
Paused trial simply shows that Lilly is doing things “by the book”
Eli Lilly’s trial was designed to test the benefits of its antibody therapy on hundreds of people hospitalized with COVID-19. The study participants also received remdesivir, another experimental drug commonly used to treat COVID-19 patients.
However, experts have stated that pauses are not unusual in large clinical trials, mentioning that declines in the health of the volunteers are not necessarily the result of the drug being tested. Rather, these halts are meant to allow independent scientific experts to review the data to determine whether or not a case was indeed related to the treatment being tested.
“This is why clinical trials are essential,” said University of Washington immunologist Marion Pepper. “The safety of the product has to be empirically proven.”
Other experts have also praised Eli Lilly and the trial’s other sponsors, including the National Institutes of Health (NIH) for halting the trials to address the safety of the product.
“They are doing things by the book,” said Yale University infectious disease physician Dr. Maricar Malinis.
The safety board is set to review the data from the trial again on Oct. 26, after which it will advise the NIH, which sponsors the trial, on whether or not it can resume. In the meantime, researchers will continue to collect data from patients who’re already enrolled in the study.
Monoclonal antibody treatments being pushed, but is it too soon?
The company is one of several pursuing experimental treatments for COVID-19 that use “monoclonal antibodies.” These are mass-produced copies of the antibodies that the immune system produces in response to the coronavirus. (Related: Possible coronavirus treatment may come from antibodies produced by… llamas?)
Antibodies can block the coronavirus from infecting healthy cells and preliminary data from testing hints that the treatments may reduce the viral load in infected people and even reduce their symptoms. In addition, Eli Lilly is also looking to see if the antibodies can protect some people from catching COVID-19 after encountering the virus.
Another company involved in monoclonal antibody research is Regeneron, the company behind the antibody treatment given to President Donald Trump after he tested positive for the coronavirus earlier this month. Trump has promoted these treatments, saying that they helped cure his condition. He has also suggested that government approval for them is imminent.
Both Eli Lilly and Regeneron have applied for emergency clearance from the Food and Drug Administration (FDA), though the former has only applied for authorization for mild and moderate COVID-19 cases, not for hospitalized patients like those in the paused trial.
The pausing of Eli Lilly’s trial, however, does raise questions over whether or not its antibody treatment should be given FDA clearance so soon. This, on top of questions of when these treatments are most effective.
Current data seems to indicate that, given too late – perhaps to a severely ill patient already in hospital – the antibodies have little effect and may even just add to the runaway immune responses that can wreak havoc on the body in the later stages of the disease.
For more on monoclonal antibodies and other new coronavirus treatments, follow Pandemic.news.
Sources include:
Tagged Under: antibody, Big Pharma, coronavirus, covid-19, disease, Donald Trump, drug safety, eli lilly, FDA, Flu, monoclonal antibody, NIH, outbreak, pandemic, Regeneron, side effects, treatment, Trials, virus